Core Technologies
Inion's foundational technology draws from the biological and mechanical principles of natural bone healing, developed over decades of working with biodegradable polymers and showcased in their exclusive Biodegradable Inion® Polymers.
The proportion of each polymer component is adjusted to match the intended application of the specific implant, ensuring that its strength, malleability, and degradation characteristics align with clinical requirements.
Our Product Range:
The Inion Bioabsorbable Implants have a wide application in orthopaedics, sports medicine, CMF, bone grafting, spine and dental. At Naton AUS, we proudly supply:
-
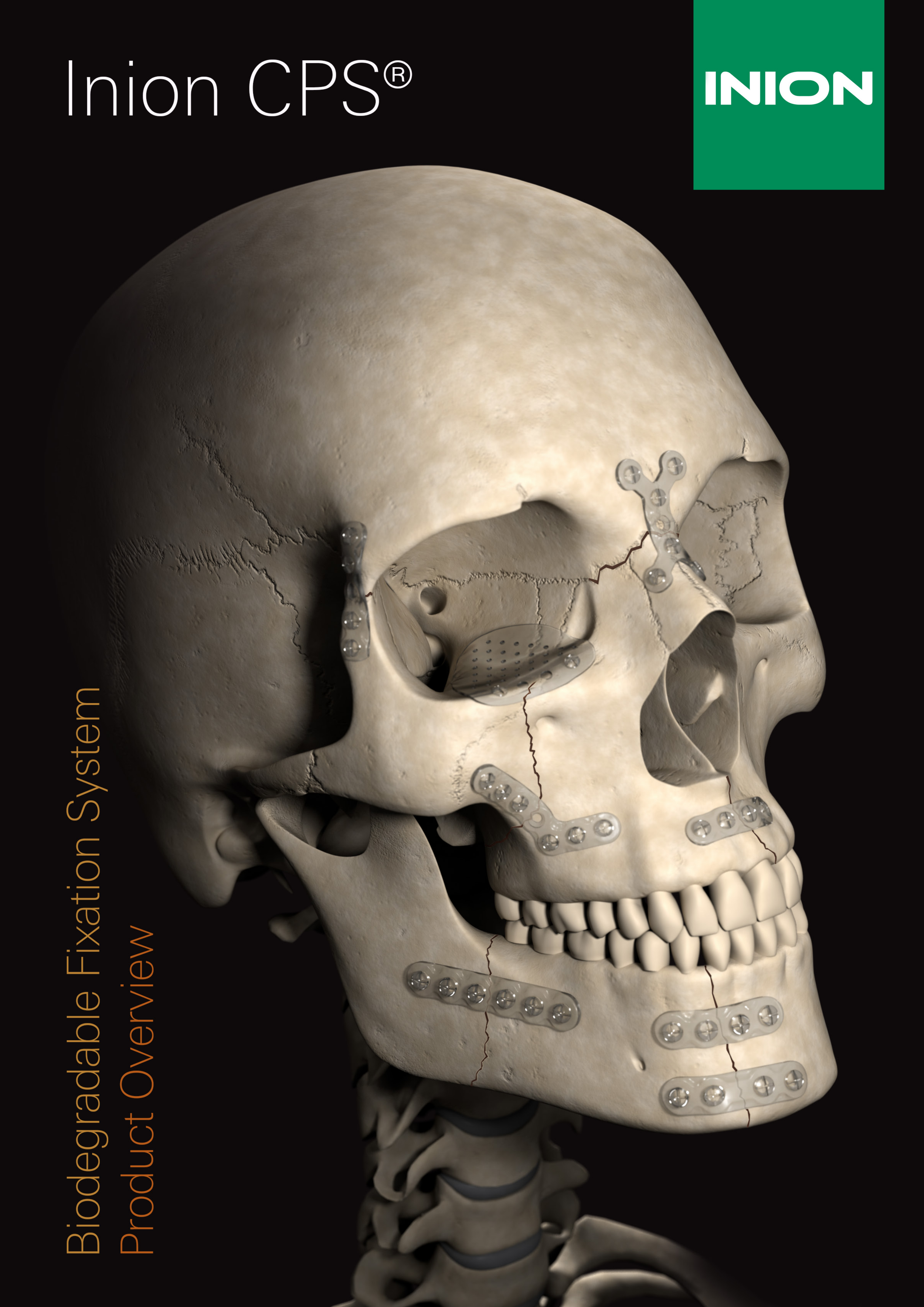
Inion CPS™ is the only bioabsorbable CMF system with applications for all areas of the facial skeleton, and comprises a range of bioabsorbable plates, screws and mesh for use in children and adults. Since their introduction in 2001, the Inion CPS™ and Inion CPS Baby™ implants have been used successfully in more than 120,000 operations by an increasing number of physicians.
-
Inion CPS Baby™ System is designed especially for the use in paediatric craniofacial trauma and reconstruction procedures. All Inion CPS™ implants are composed of Inion® biodegradable polymer blends.
-
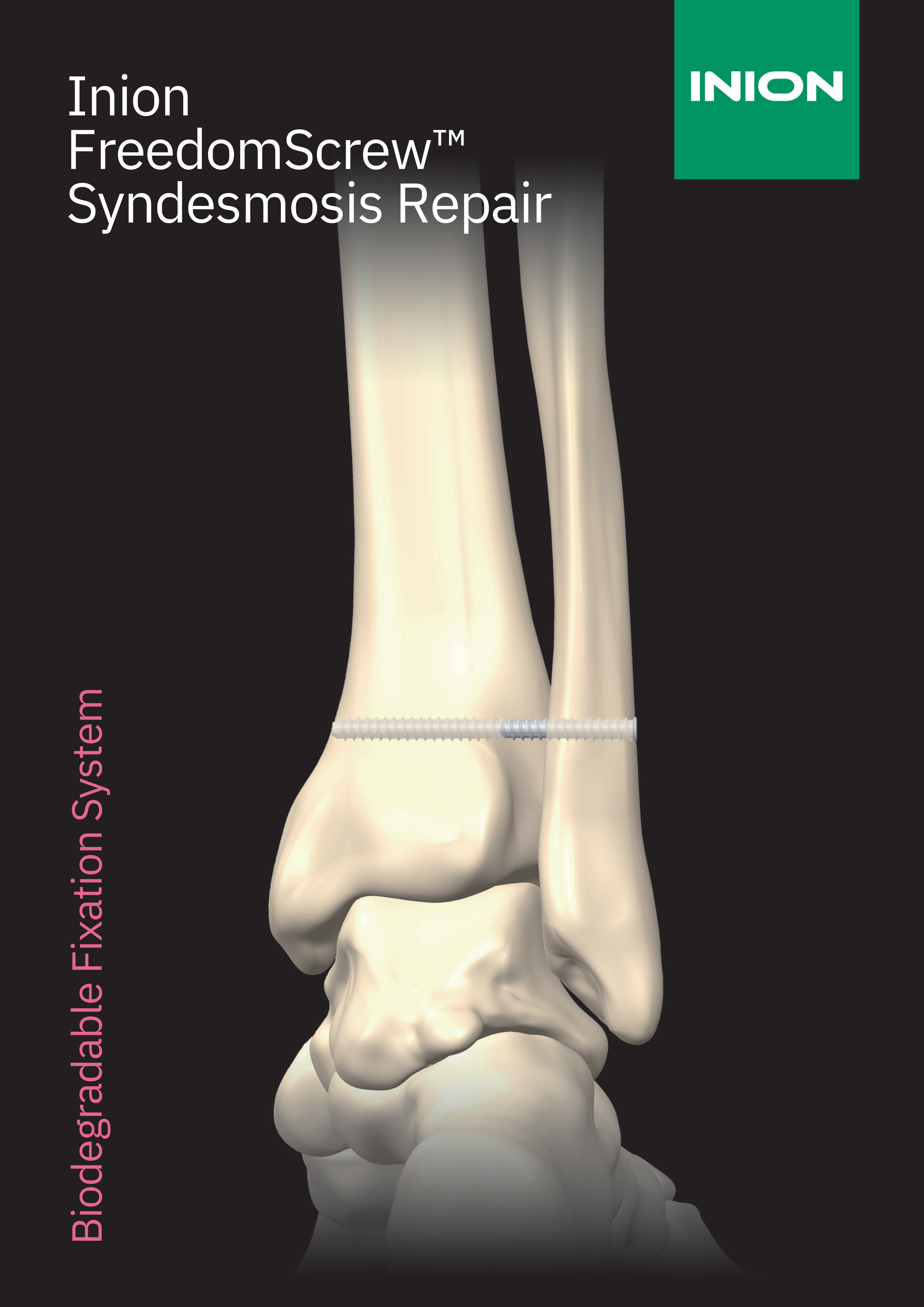
Inion FreedomScrew™ is a strong and fully versatile resorbable screw for orthopaedic fixations. Because of its unique manufacturing method and intelligent technical properties, Inion FreedomScrew™ offers many clear benefits for the operating surgeon who appreciates easiness, reliability and versatility from the implant.
-
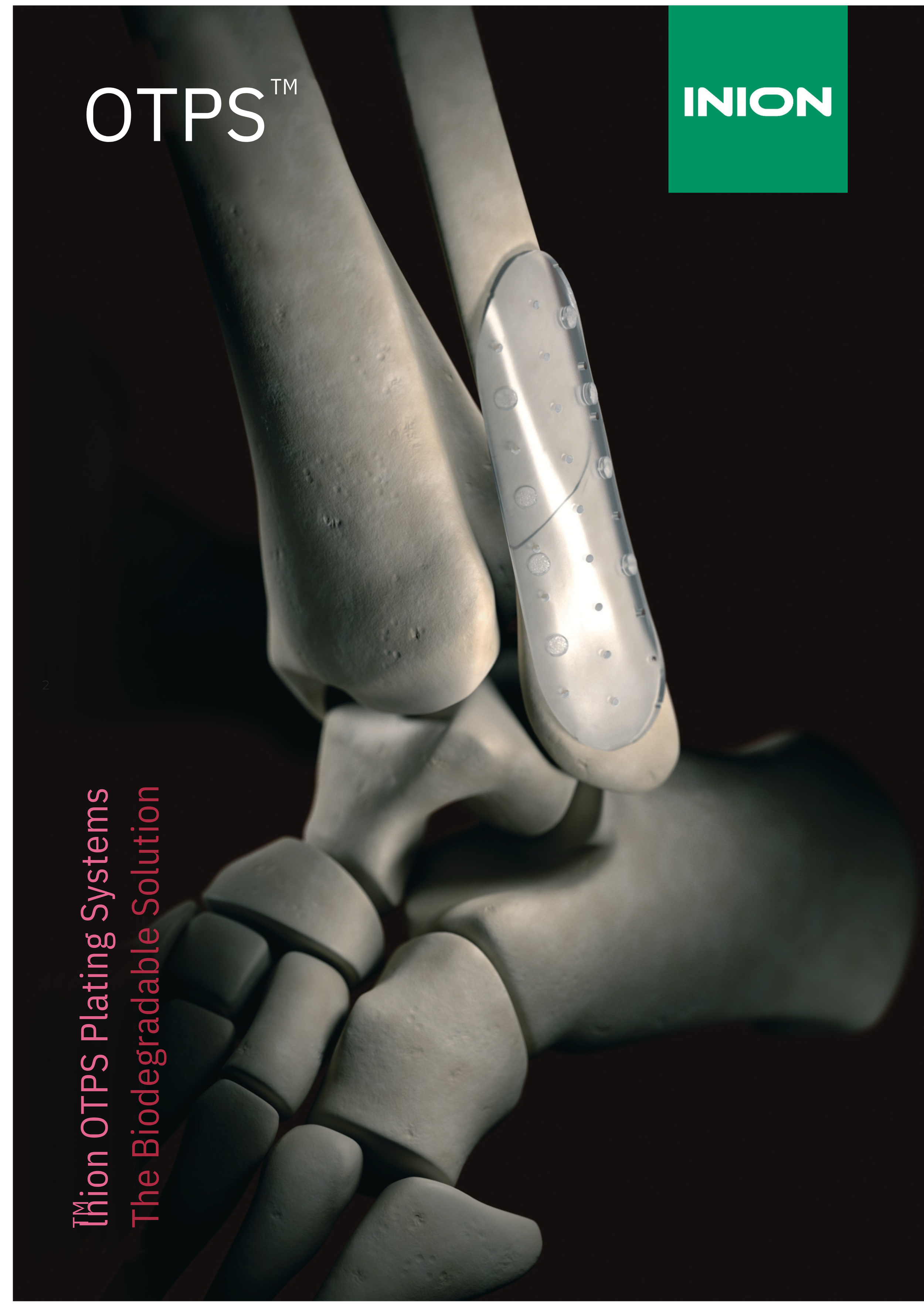
Inion OTPS™ Biodegradable Ankle Plating System is a versatile fracture fixation system. The system includes a fully customisable plate which contours to all anatomies, and fixation screws which can be implanted polyaxially to provide the best possible fixation.
-
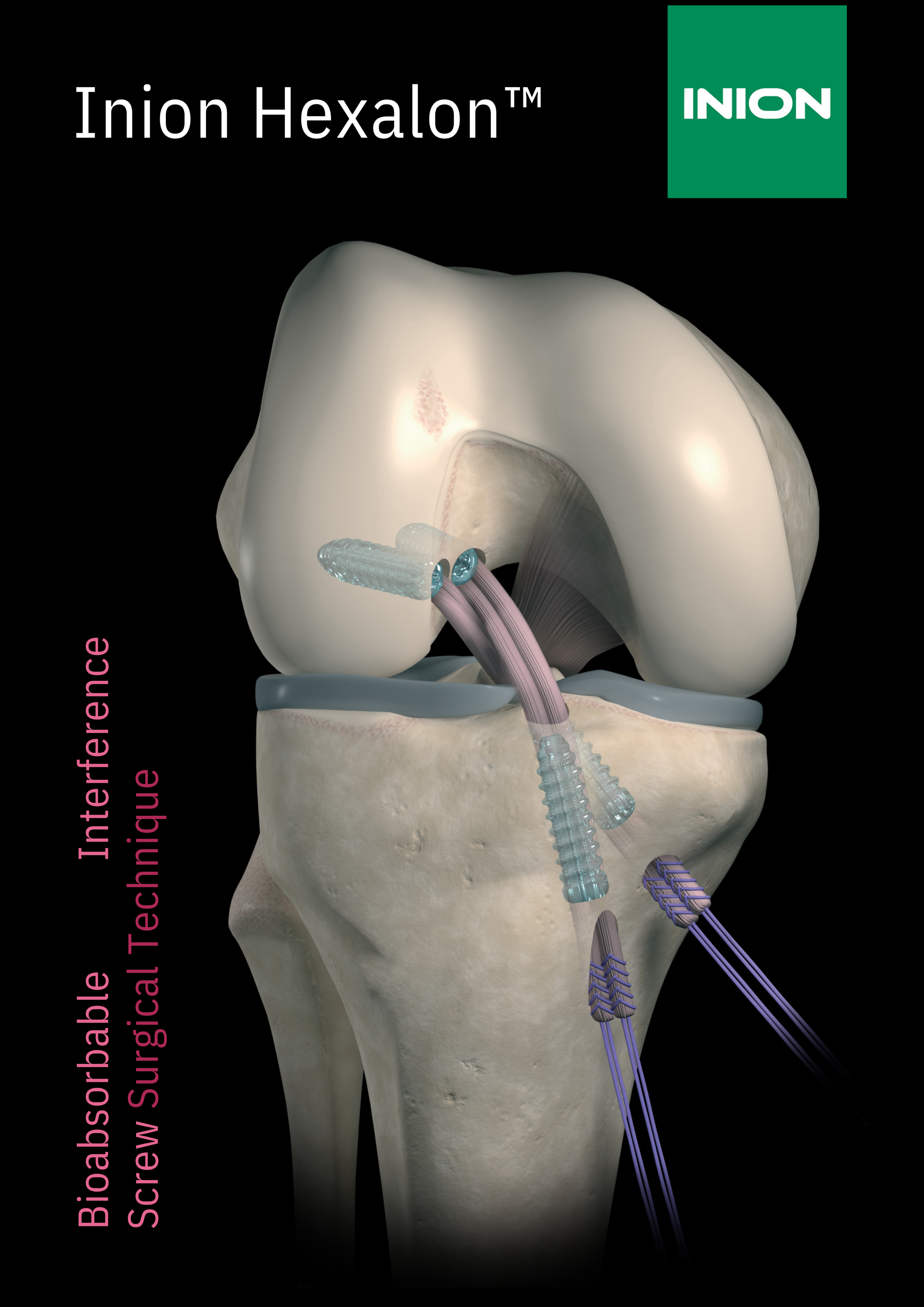
Inion Hexalon™ is the first coloured interference screw that allows clear visibility during arthroscopic insertion. In addition to traditional single-bundle procedures, Inion Hexalon™ screws can also be used for the fixation of soft tissue tendon grafts in double-bundle anterior cruciate ligament reconstruction.
Find out more:
Inion CPS™
-
Suited for all CMF areas, including:
Paediatric craniofacial trauma and reconstruction
Fractures and reconstructive procedures of the cranium
Orthognathic surgery and trauma of the mid-face and maxilla
Fractures and osteotomies of the mandible
Download Inion CPS Brochure
Inion CPS Baby™
-
Inion CPSTM Baby System is designed especially for the use in paediatric craniofacial trauma and reconstruction procedures.
Download Inion CPSBaby Brochure
Inion OTPS™
-
Inion OTPS™ Ankle Plating System
General indications: Maintenance of reduction and fixation of cancellous bone fractures, osteotomies or arthrodesis of the upper extremity, ankle and foot in the presence of appropriate plaster cast immobilisation.
Specific indications:
- Fractures and osteotomies of the malleoli
- Ankle fractures
Inion OTPS™ Mini System
Intended for fixation of non-comminuted diaphyseal fractures of the metacarpal, proximal phalangeal, middle phalangeal and osteotomies in the presence of appropriate immobilisation.
Inion OTPS™ Mesh System
Intended to sustain the relative position of weak bony tissue, e.g., bone grafts, bone graft substitutes, or bone fragments from comminuted fractures.
Indicated for cement restriction in total joint arthroplasty procedures.
Only when used in conjunction with traditional rigid fixation, these implants are intended to sustain the relative position of weak bony tissue in trauma and reconstructive orthopaedic procedures involving the following:
- Long bones
- Flat bones
- Short bones
- Irregular bones
- Appendicular skeleton - Thorax
When used alone (without traditional rigid fixation), these implants are intended to maintain the relative position of bone grafts or bone graft substitutes in reconstructive orthopaedic procedures involving:
- Tumor resections where bone strength has not been compromised
- Iliac crest harvest sites
Download Inion OTPS Brochure
Inion FreedomScrew™
-
The Inion FreedomScrewTM is intended for maintenance of alignment and fixation of bone fractures, comminuted fractures, osteotomies, arthrodesis or bone grafts (i.e., autografts or allografts) in the presence of appropriate additional immobilisation (e.g., rigid fixation implants, cast or brace).
In addition, the Inion FreedomScrewTM 3.5/4.0/4.5 mm products are specifically intended for use in following indications:
General indications: maintenance of reduction and fixation of cancellous bone fractures, osteotomies or arthrodesis of the upper extremity, ankle and foot in the presence of appropriate brace and/or immobilization.
Specific indications: fractures and osteotomies of the malleoli, and ankle fractures.
Download Inion FreedomScrew Brochure
Inion Hexalon™
-
The Inion Hexalon™ bioabsorbable interference screw is intended for:
EU: interference fixation in anterior cruciate ligament
reconstruction using soft tissue grafts.
US: soft tissue (including ligament, tendon, and bonetendon-bone graft) fixation to bone in surgeries of the
knee, shoulder, elbow, ankle, foot, hand and wrist where
the offered screw sizes are patient appropriate.
Inion Hexalon™ is contraindicated in the following cases:
■ Insufficient quality or quantity of bone for interference
screw attachment
■ Active or potential infections
■ Patients with known allergy to the implant constituents
or its degradation products
■ Patient conditions including limited blood supply,
chronic disease which causes insufficient quality of
bone, and where patient co-operation cannot be
guaranteed (e.g. alcoholism, drug abuse).
Download Inion Hexalon Brochure